Shop
Showing all 4 results
BPB Protamine Monoclonal Antibodies
A new set of protamine monoclonal antibodies (MAbs) have been developed that recognize and bind to protamines P1 and/or P2. Similar to the Hup antibodies, these new MAbs recognize and bind to the isolated protamines and those in their native state (while bound to DNA in spermatids and sperm).
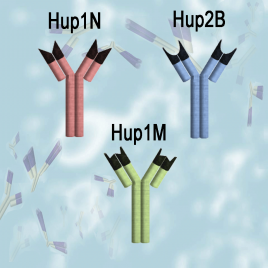
HUP Protamine Monoclonal Antibodies
Hup1N, Hup2B and Hup1M anti-protamine monoclonal antibodies are provided as affinity purified reagents. Hup1N and Hup2B recognize and bind to protamine P1 and P2 molecules, respectively, from many types of mammals. Hup1M is selective for human protamine P1 only.
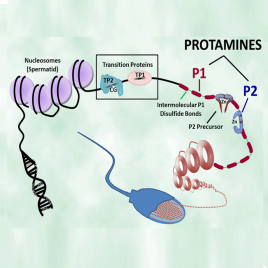
Mammalian Protamines
Native isolated and synthetic forms of the mammalian protamine P1 and P2 proteins are available
- Native isolated protamine molecules:
- Protamine P1 as isolated from sperm (bull, boar, ram)
- Protamine P1 isolated from sperm and then purified by HPLC (bull and hamster)
- Protamine P1P2 Mix as isolated from sperm (hamster, stallion, and human)
- Synthetic HPLC purified human, stallion and mouse protamines
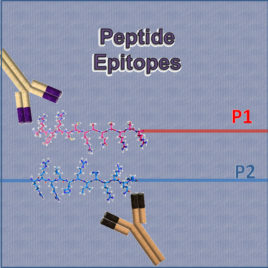
Protamine Epitopes
These synthetic peptides contain the protamine P1 and protamine P2 epitopes recognized by the antibodies Hup1N and Hup2B, respectively.